Atomic orbitals#
What you need to know
A wavefunction for a one-electron system is called an orbital. For an atomic system such as H (hydrogen atom), it is called an atomic.
The orbital plots demonstrated the shapes of the orbitals but this does not tell us anything about the radial extent (i.e., how far the orbital reaches).
As the value of \(Z\) is increased, the radial extent decreases. This indicates that for higher nuclear charge, the electrons will reside closer to the nucleus.
The radial wavefunctions have \(n - l - 1\) nodes between distances from zero to infinity.
The angular part of wavefunctions ahve \(l\) nodes
The total number of nodes is \(n-1\)
Definition of Atomic Orbital
Atomic orbital (AO): single–electron wavefunction (exact for H‐like atoms)
Principal quantum number: \(n=1,2,3,\ldots\)
Orbital angular momentum: \(\ell=0,1,2,\ldots,n-1\)
Magnetic quantum number: \(m_\ell=-\ell,-\ell+1,\ldots,\ell\)
Probability distribution (full 3D):
Radial probability distribution
Angular probability density
Notes:
\(s\) orbitals \(\ell=0\) are isotropic: \(P_\Omega=\tfrac{1}{4\pi}\).
\(p,d,\ldots\) show angular nodes where \(Y_{\ell m_\ell}=0\), setting orbital shapes.
Radial profiles of atomic orbitals#
When visualizing the radial probabilities, it is possible to do directly plot the square of the radial wavefunction (\(R_{nl}^2\)) or the radial probability density (\(P_{nl}\)):
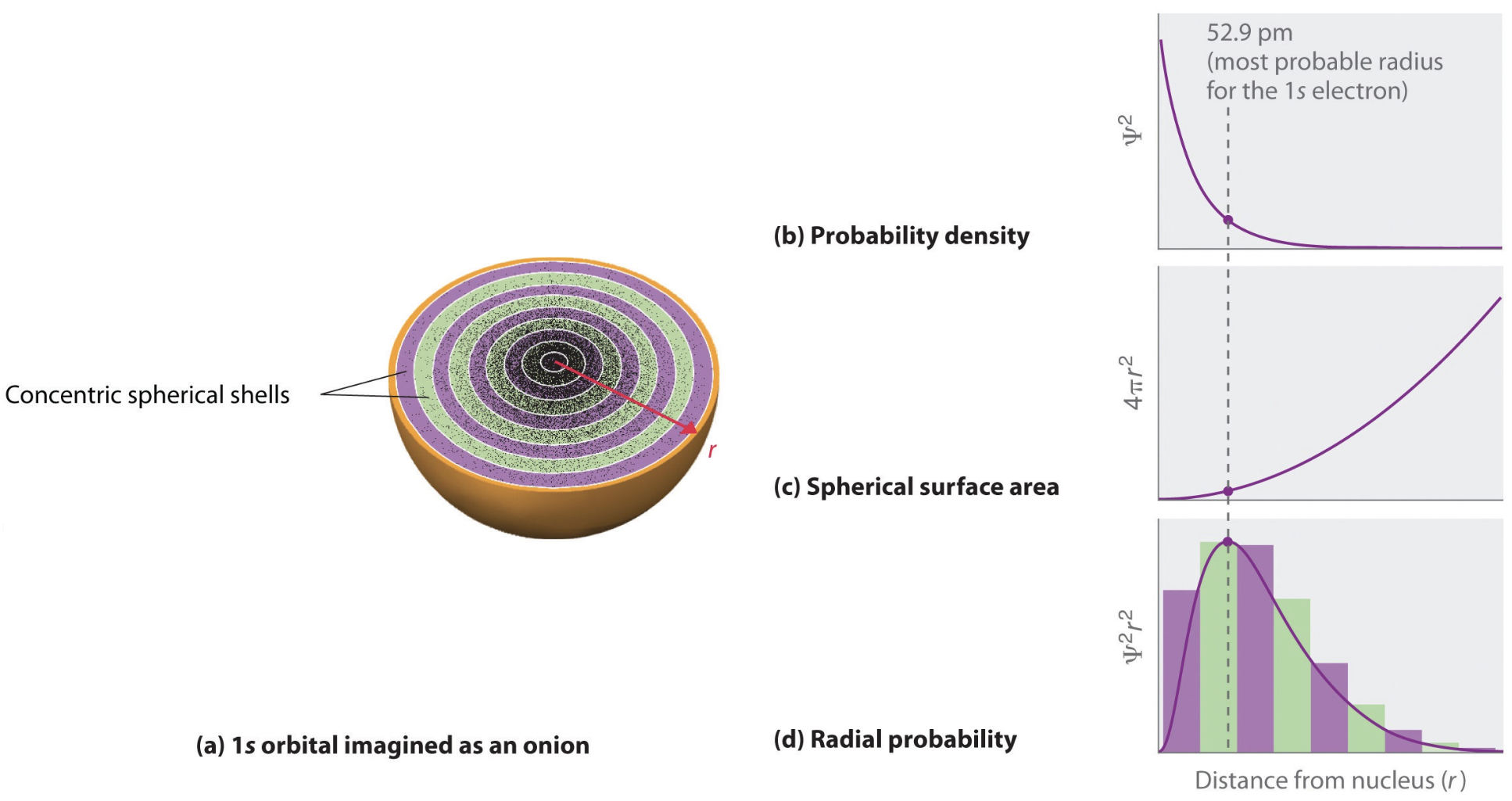
According to this expression, the most probable radius for an electron on hydrogen atom \(1s\) orbital is \(a_0\) (the Bohr radius). Previous figures showed examples of \(R_{nl}\) and \(P_{nl}\). Probability densities are useful, for example, in understanding charge distributions in atoms and molecules.
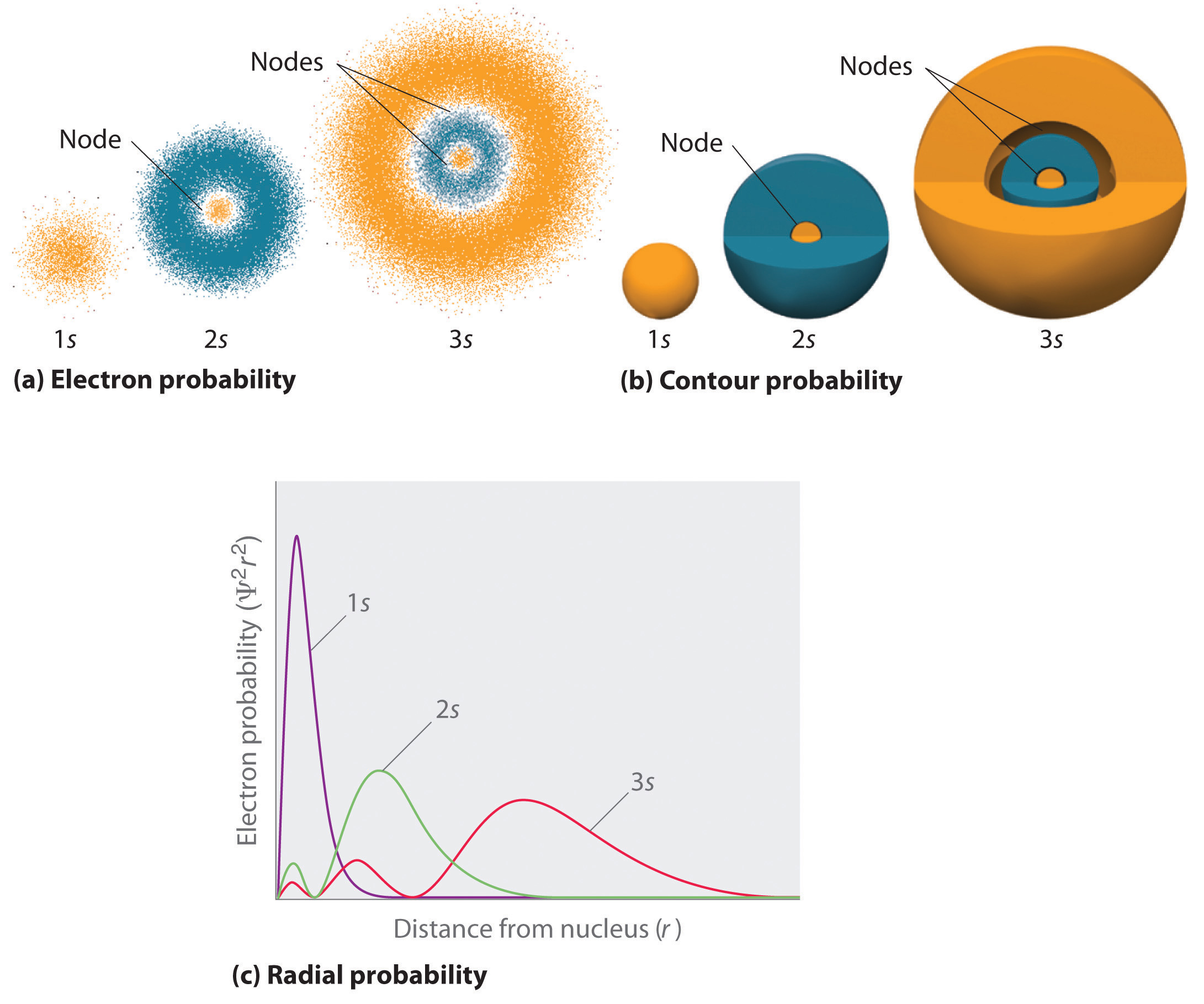
As the principal quantum number \(n\) increases, the electron moves out to greater distances from the nucleus. The average distance for an electron in a given orbital (with quantum numbers \(n\) and \(l\)) is given by (this is not the expectation value):
Note that the expectation value of \(r\) and the most probable value for \(r\) are not equal. The expectation value can be thought of like an average and the most probable value like a maximum value.
3D shapes of orbitals#
For degenerate states with \(l > 0\), we have an additional degree of freedom in choosing how to represent the orbitals. In fact, any linear combination of given \(3l\) orthogonal eigenfunctions corresponding to a degenerate set with orbital angular momentum \(l\), is also a solution to the Schrodinger equation.
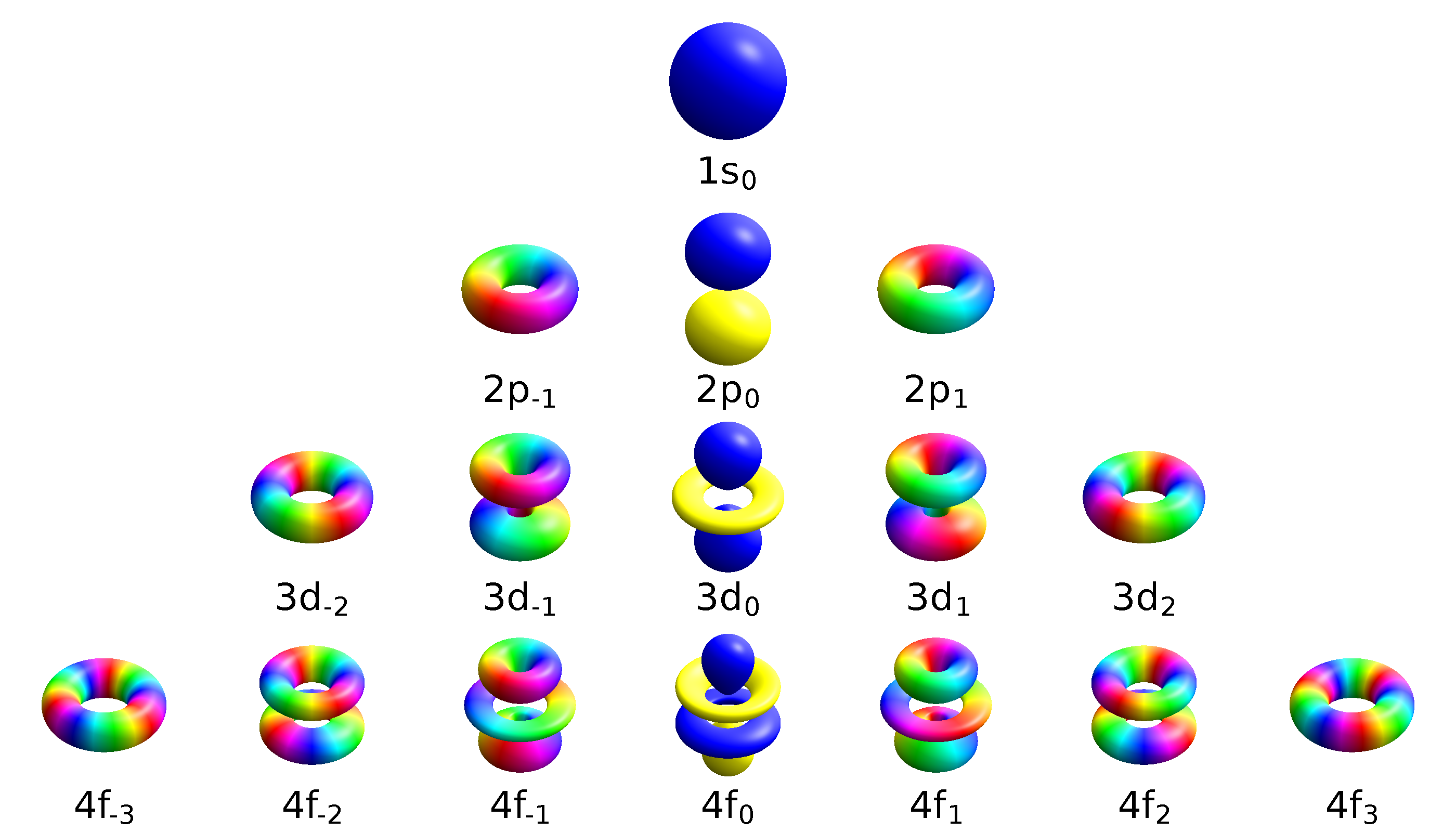
Two commonly used representations are the Cartesian form, which are real valued functions and have been, in the case of \(l = 1\), denoted by \(p_x\), \(p_y\) and \(p_z\), and the eigenfunctions of the angular momentum (\(L^2\) and \(L_z\)), which are complex valued and are denoted by \(p_{-1}\), \(p_0\) and \(p_{+1}\). The relation between the representations is:
Both polar and cartesian forms represent the same physical electron density: just in different coordinate frames!
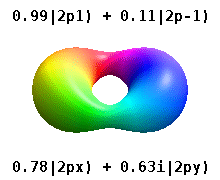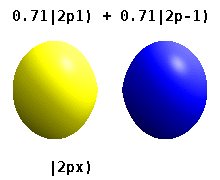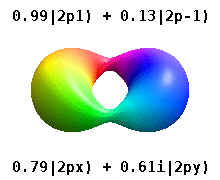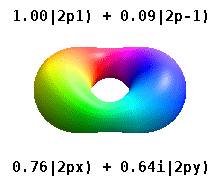
Note by combining \(p_x\), \(p_y\) and \(p_z\), the lobe of the orbital can be made to point at any direction. For \(d\)-orbitals, we have five degenerate levels:
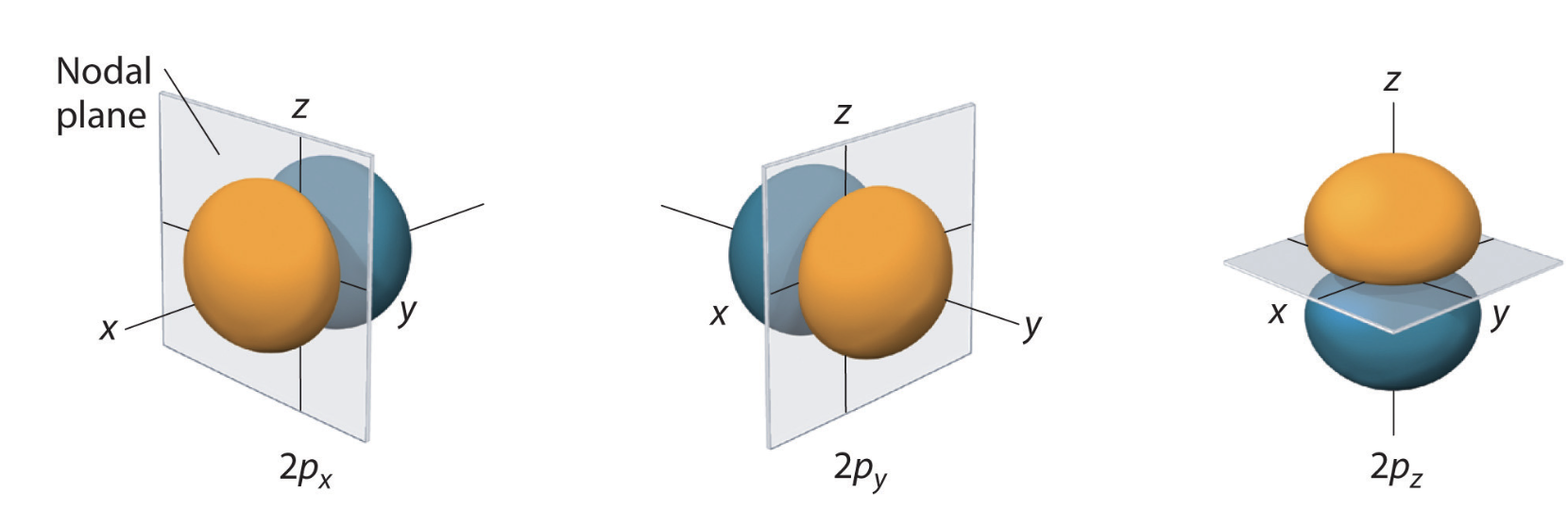
Table of 2D orbitals#
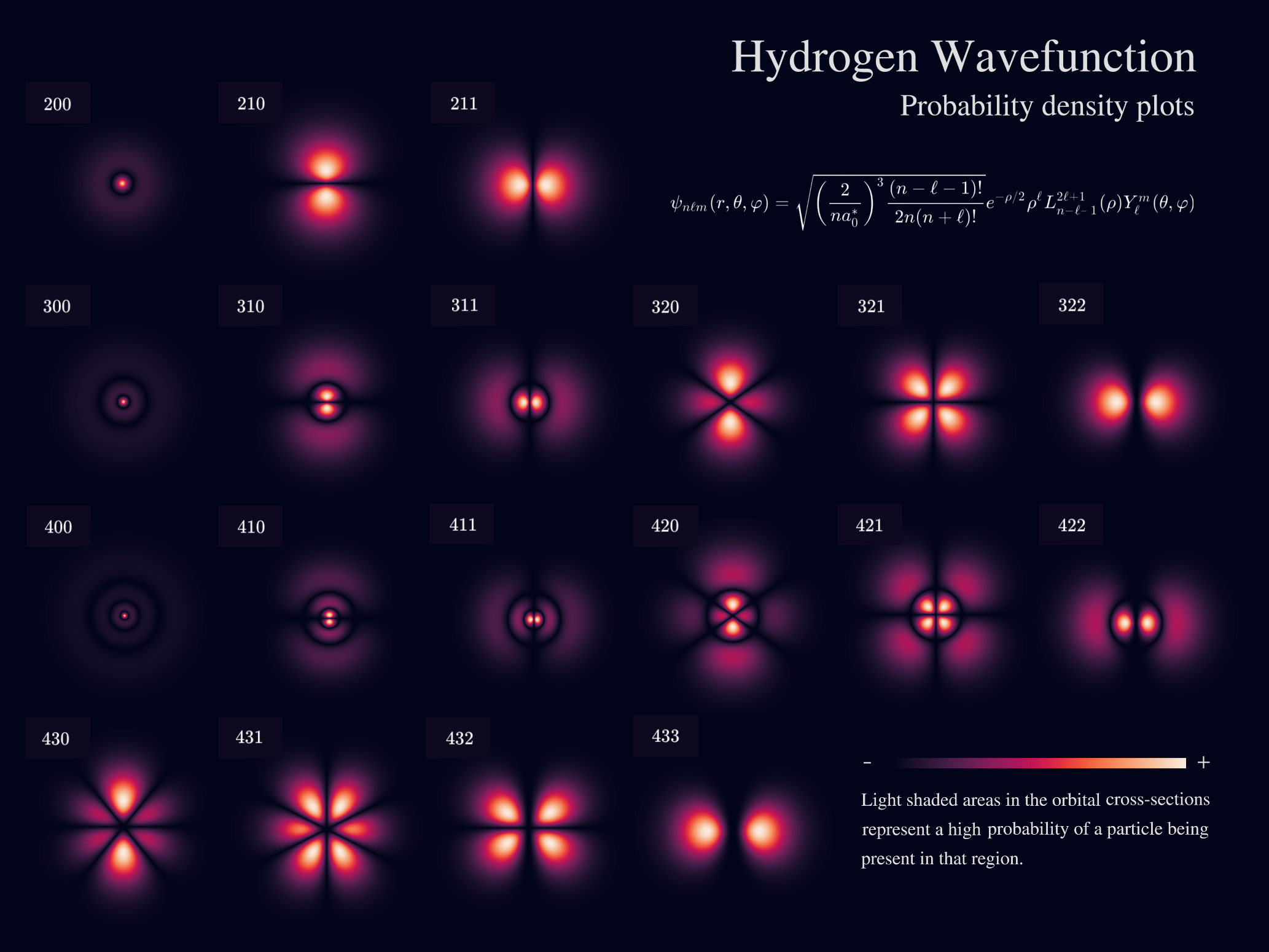
Table of 3D orbitals#
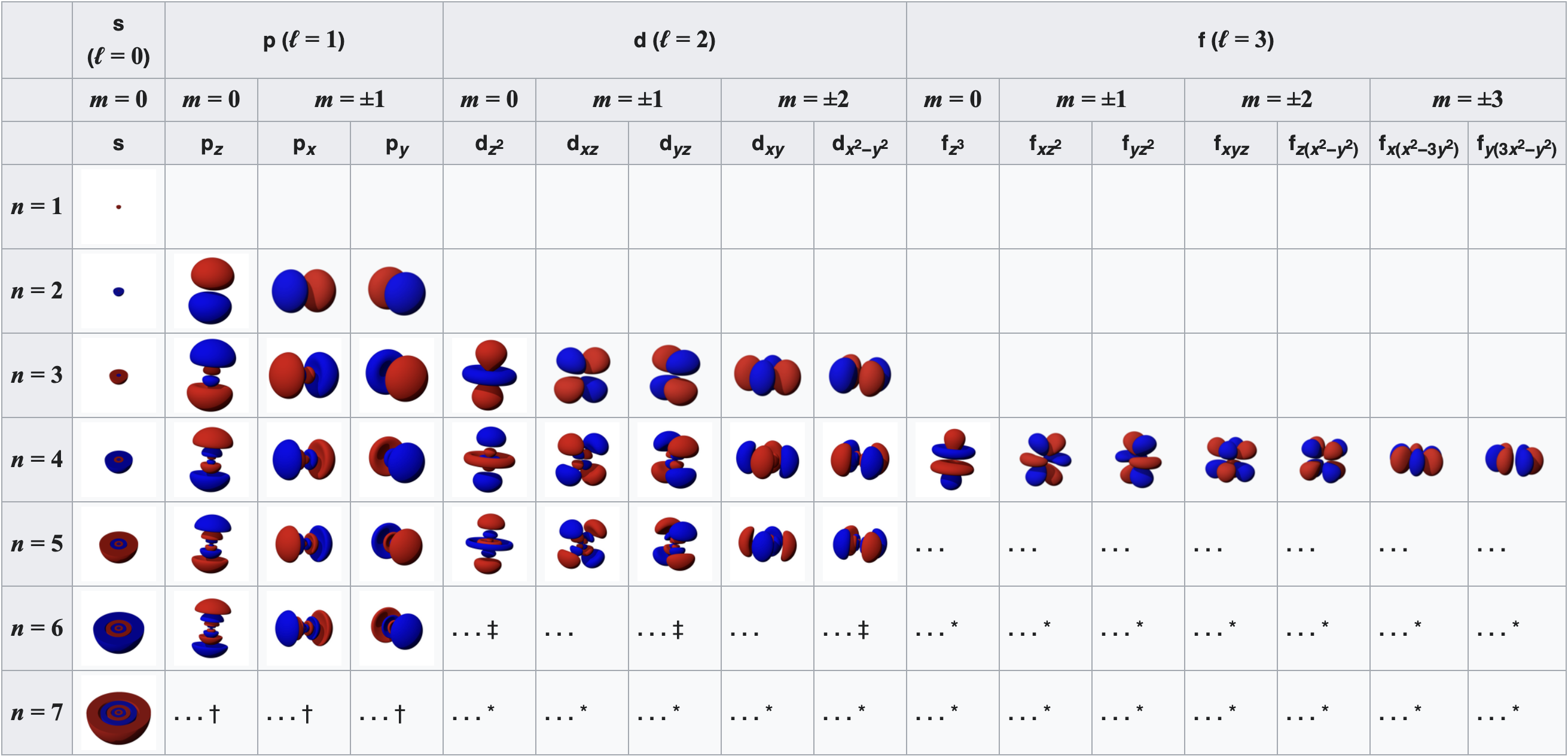
Interactive plotter of Atomic Orbitals#
Problems#
Problem 1#
For the hydrogen atom, how many possible quantum states correspond to the principal number \(n=3\)
What are the energies of these states?
Consider several values for n, and show that the number of orbitals for each \(n\) is \(n^2\)
Solution
If n=3 the allowed values of l are 0, 1, and 2.
If \(l=0;\, m=0\) (1 state)
If \(l=1;\, m=−1,0,1\) (3 states);
If \(l=2;\, m=−2,−1,0,1,2\) (5 states).
In total, there are 1 + 3 + 5 = 9 allowed states. This confirms that number of orbitals for H-atom is \(n^2\)
Because the total energy depends only on the principal quantum number! n=3. the energy of each of these states is given by:
Problem 2#
The notation \(3d\) specifies the quantum numbers for an electron in the hydrogen atom. What are the values for \(n\) and \(l\)?
What are the values for the energy and angular momentum?
What are the possible values for the magnetic quantum number?
What are the possible orientations for the angular momentum vector?
Hint
3d orbital corresponds to n=1 and l=2. A number of \(m_l\) values are possible
Orientation of \(L_z\). with respect to L is defined by \(cos\theta = L_z/L\)
Problem 3#
Locate nodes or nodal surfaces of \(3p_z\) orbital:
Solution
\(3p_z\) corresponds to \(n=3\), \(l=1\) and \(m_l=0\)
Radial part of the equations is zero at \(r=0\) and \(r=6a_0\). First one is part of a boundary condition and not a node becaue wavfunction is not changing a sign. Hence 1 node from radial part.
Angular part has one nodal surface due to \(cos\theta=0\) which happens at \(\theta=\frac{\pi}{2}\)
We have 2 nodes for \(3p_z\) orbital. The toal number of nodes are n-1 where one has l angular nodes and n - l - 1 radial nodes.
Problem 4#
Calculate average distance from nucleus to find the electron in H-atom in its ground state.
Calculate probabiltiy to find the electron within first Bohr raidus \(a_0.\)
calcualte the most probable value of r to find the electron.
Solution
Ground state is 1s;
Compute averages. Averages or expectations of observables in quantum mechanics are calculated as usual by \(\langle\psi |A| \psi \rangle\) expression:
The last integral is evaluated using the following table integral:
Computing probabilities Wavefunction square is a probability distribution function to find electron in small volume dV in 3D space. Probability to find electron at a distance regardless of angles is found by integrating out angles:
Probability to find electron in a range \(0\leq r\leq a_0\) is found by integrating (summing probabilities) over that range:
Most probable value is found by finding the maximim value of \(P(r)\)
Carrying out the derivative we find that \(r_max=a_0\)
Problem 5#
Show that \(|210\rangle\) is normalized and orthogoanal to \(|200\rangle\)
Hint
This problem is solved in Chapter 7 of McQuarrie’s book. Page 326
Problem 6#
Calculate average potential energy of H-atom in its ground and first excited states.
Hint
This problem is solved in Chapter 7 of McQuarrie’s book. Page 3332